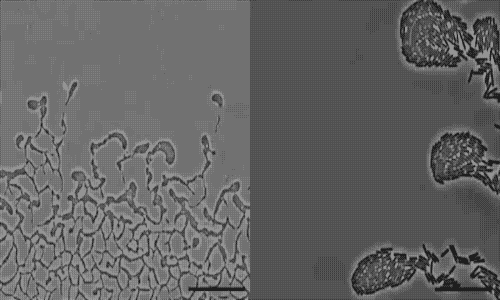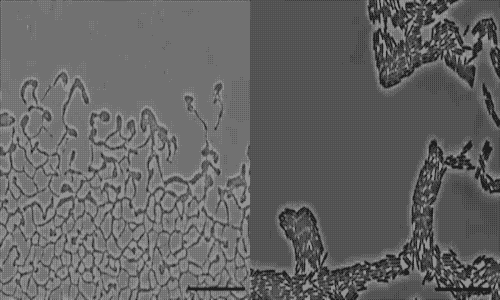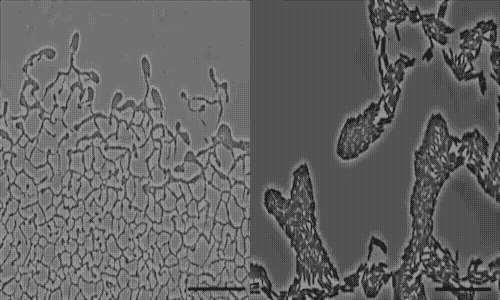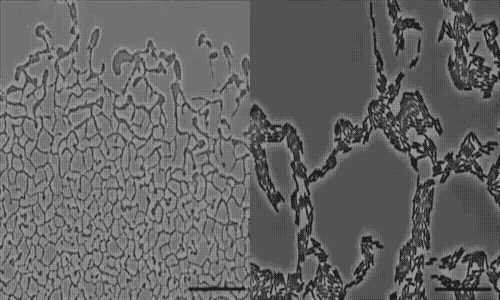
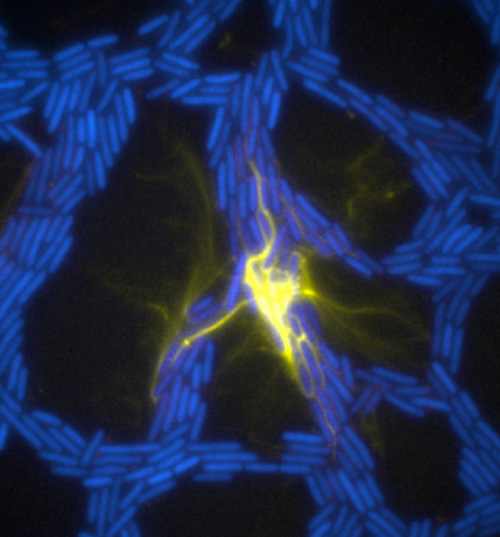
The team used a number of imaging technologies including GE’s DeltaVision OMX Blaze microscope equipped with high-speed cameras. They observed for the first time how bits of DNA that stick to the outside wall of the bacteria hold the biofilm together and guide its movement. Scientists call this microbe glue extracellular DNA, or eDNA. “We knew it was there - we had evidence from observing how the bacteria were behaving - but we just couldn’t see it or detect it in any way,” Whitchurch says. “The high-sensitivity cameras on the OMX Blaze enabled us to answer the question of how the bacteria were gluing themselves together.”
The researchers released their findings in the current issue of the Proceedings of the National Academy of Sciences. They observed cell movements during the expansion of living biofilms by combining microscope images with sophisticated computer-vision algorithms. Their analysis found “highly coherent groups of bacteria” at the leading edges of the biofilm. These groups create channels that help cells following behind them migrate, and leave eDNA that “facilitates efficient traffic flow” throughout the channel network.
 Francis Collins, director of the National Institutes of Health, agreed. He called images produced by the OMX “showstoppers.”
Francis Collins, director of the National Institutes of Health, agreed. He called images produced by the OMX “showstoppers.”Delta Vision OMX is for research use only, not for use in diagnostic procedures. Delta Vision OMX is a GE trademark.
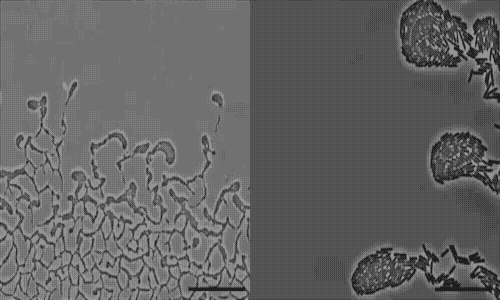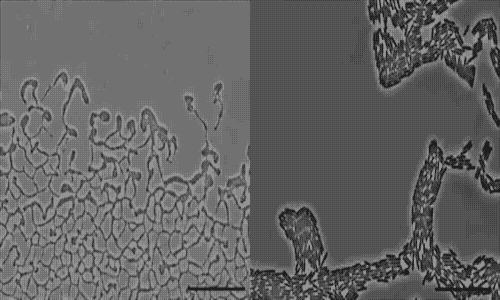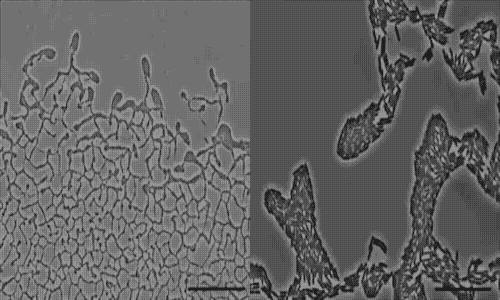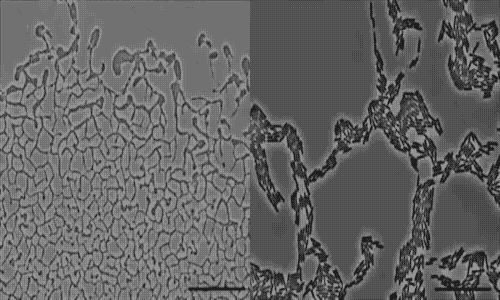 actively colonize surfaces via a process called twitching motility.
actively colonize surfaces via a process called twitching motility.