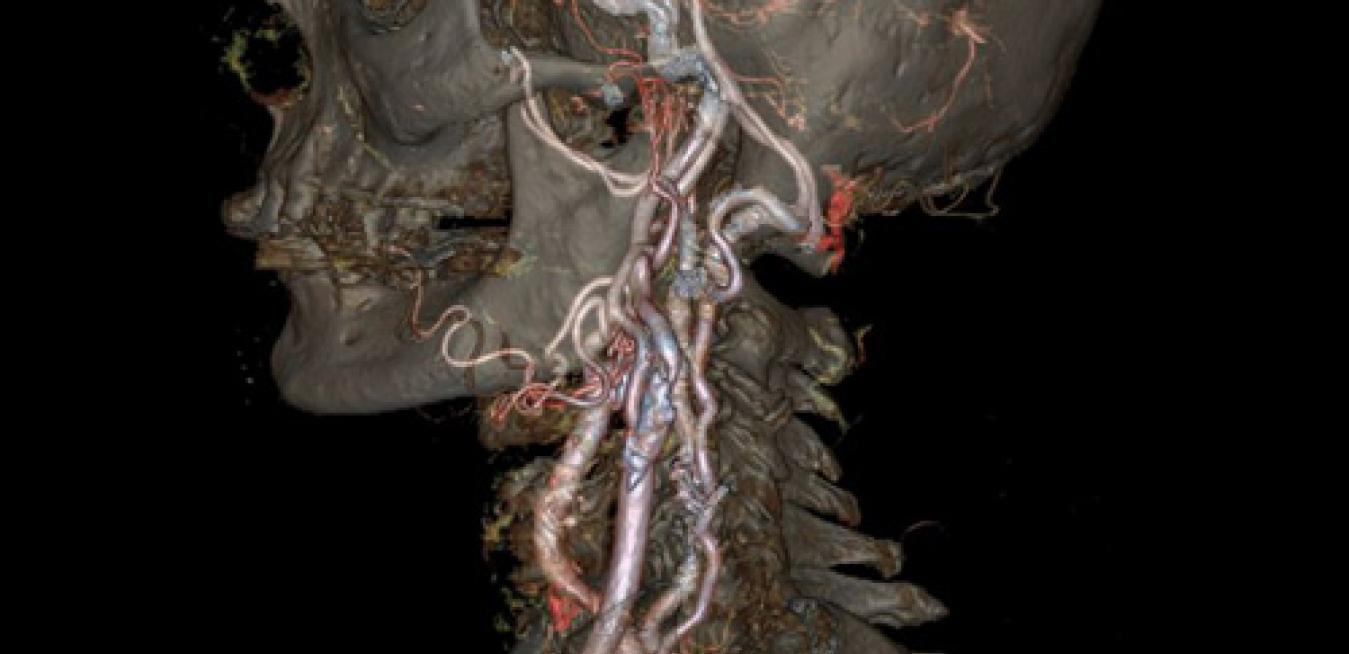
Putting interventional radiologists in the center of their procedures
Wauwatosa, WI -- April 30, 2014 -- GE Healthcare announced today it has received Food and Drug Administration (FDA) clearance for its Discovery* IGS 740, a new rail-free mobile angiography system with a 41x41-cm detector. The Discovery IGS 740 is the first of this kind of mobile angiography system in the industry to receive FDA approval.