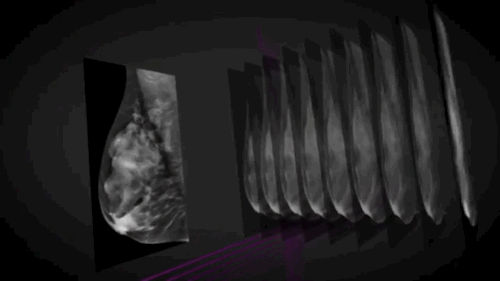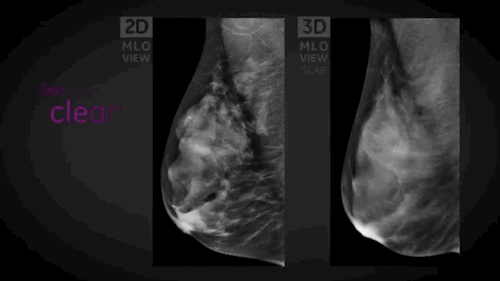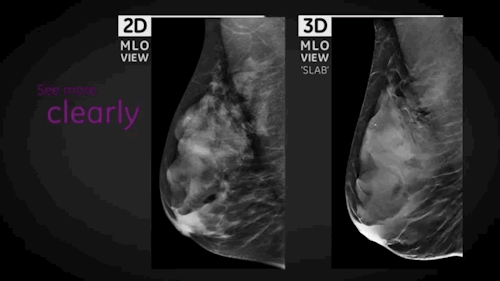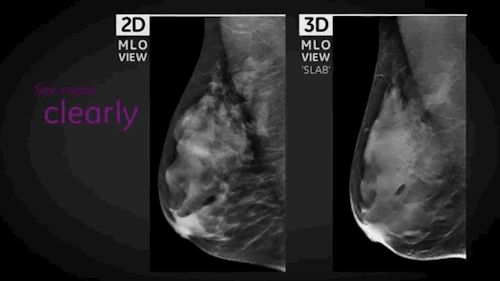
Wauwatosa, WI, September 3, 2014 --- GE Healthcare (NYSE: GE) today announced the FDA approval of SenoClaire*, GE's new breast tomosynthesis solution designed with a three-dimensional imaging technology. In collaboration with Massachusetts General Hospital, GE developed SenoClaire technology that uses a low-dose short X-ray sweep around the positioned breast with nine exposures acquired with a "step-and-shoot" method, removing the potential motion from the tube helping to reduce blur and increase image sharpness.
In 1965, French radiologist Charles Gros built the first X-ray machine dedicated to screening breasts and effectively launched mammography as a viable breast cancer test. The machine, which was built by Thomson CGR, used a special X-ray tube developed by his colleague Emile Gabbay. It was made from molybdenum and emitted low-energy radiation that produced uniform images and contrast that allowed doctors to see breast tissue in greater detail.
Vienna -- 6th March 2014 -- GE Healthcare, a unit of GE (NYSE: GE), today announced the launch of its 'Dose Blueprint' in Europe at European Congress of Radiology (ECR) in Vienna, a programmatic approach to lowering dose across the entirety of a hospital. The launch coincides with the recently published European Directive which includes specific measures regarding the use of radiation for medical treatments and diagnostics.
business unit
tags
Vienna -- 6th March 2014 -- GE Healthcare, a unit of GE (NYSE: GE), today announced at the European Congress of Radiology (ECR) in Vienna the findings of a new European study showcasing compelling evidence around the value predictive maintenance service software offerings can bring for clinicians, hospitals and patients.