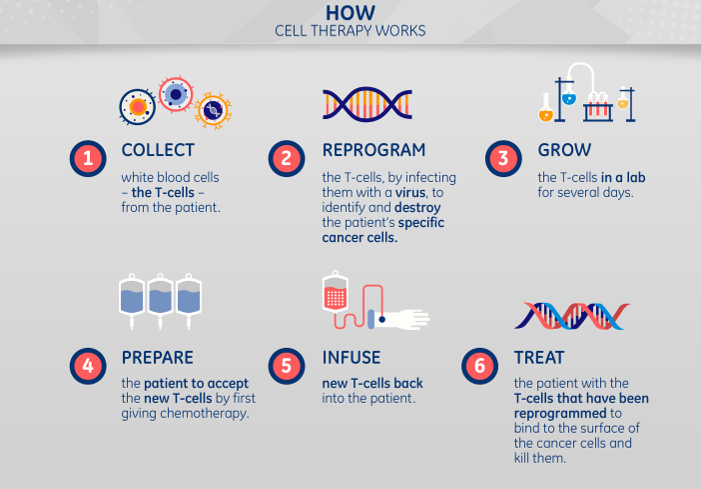
But her parents kept fighting, and in April 2012 enrolled Emily in an experimental therapy pioneered at the Children’s Hospital of Philadelphia. The treatment involved harvesting her own T cells, a type of white blood cells that help guide the body’s immune response by targeting foreign cells in the bloodstream.
The Philadelphia team first genetically reprogrammed a handful of the T cells to attack the cancer in her blood. Next, they multiplied the cells in a lab and infused them back into her body. Emily’s fortified immune system engaged the disease and she went into remission three weeks later. Now 12 years old, Emily’s cancer has been in remission since May 2012.
Live-cell treatments have emerged as one of the most promising avenues for cancer treatment. A recent study in the Journal of Oncology showed that 70 percent of adult leukemia patients had their tumors shrink or disappear after treatment similar to what Emily received, known as CAR T cell therapy. Last year the FDA approved a CAR T cell therapy for children suffering from acute lymphoblastic leukemia who fail to respond to other treatments, and a similar treatment for adults with advanced lymphoma. Trials of live-cell treatments are underway for other kinds of cancers as well.“We’re entering a new frontier in medical innovation with the ability to reprogram a patient’s own cells to attack a deadly cancer,” FDA commissioner Scott Gottlieb said last year.
 “Cell therapy has the potential to cure everything from cancer to diabetes,” says GE's Phil Vanek. “But we need to make it affordable and scalable.” Top and above images credit: GE Healthcare Life Sciences.
“Cell therapy has the potential to cure everything from cancer to diabetes,” says GE's Phil Vanek. “But we need to make it affordable and scalable.” Top and above images credit: GE Healthcare Life Sciences.The next battle involves moving this promising therapy out of the lab. As live-cell treatments take off in the next few years, lowering costs and maintaining consistent quality will pose a unique challenge to the industry. Live-cell treatments are difficult to scale: By nature, they are tailored to the individual patient. The need to transport a patient’s own living cells to a production facility where they can be genetically modified, and then return them to the doctor’s office or hospital, poses a far greater logistical challenge than transporting conventional biological materials like proteins and antigens — doctors can’t just freeze-dry cells and add water later. “For live cells, cryogenics is the only practical option,” says Phil Vanek, general manager of cell therapy strategy at GE Healthcare.
That’s why last year his employer acquired Asymptote, a U.K.-based startup developing cryogenic technologies for freezing, preserving and transporting cells. Among other investments in the cell therapy space, GE is part of Toronto’s new Center for Advanced Therapeutic Cell Technologies, which opened last September. The center is designed for new processes that could enable companies to offer cell therapy to millions of patients. “Cell therapy has the potential to cure everything from cancer to diabetes,” Vanek said. “But we need to make it affordable and scalable.”
Cryogenics is the technology of the deep freeze — the realm of liquid nitrogen, 196 degrees Celsius below zero. Liquid nitrogen was the gold standard in cryogenics for decades, but it is too blunt an instrument for the freezing and thawing of live cells for medical treatments.
Fortunately, an alternative has emerged from work that NASA and the U.S. military have done to keep their space-bound sensors cool. They used state-of-the-art materials and engineering to update a Scottish invention called a Stirling engine, invented in 1816 by Robert Stirling. The 21st-century version uses a configuration of pistons and gears to pump heat out of a chamber.
Asymptote, now part of GE Healthcare, has incorporated the Stirling engine into a suite of instruments that can freeze and thaw living cells in a tightly controlled way. The devices also provide data on the temperature of the cells throughout their journey from patient to lab and back again.
Asymptote founder John Morris froze T cells for the first time as a Ph.D. student in the 1970s at the University of London. He came to appreciate the cell as a kind of biochemical machine: freeze it, and the machinery comes to a standstill; thaw it, and the machinery picks up where it left off. “At the time,” he says, “nobody was interested.” That changed about 10 years ago, with the rise of live-cell therapies. “People realized that they had to freeze cells correctly. Each sample you freeze has to be the same as the last one.”
Last month, Innovate UK, the government’s innovation agency, awarded funding to several National Health Service hospitals to partner with industry players, including Morris’ Asymptote team, to coordinate a strategy to increase the capacity of the NHS to develop systems and processes to deliver these advanced therapies to patients. The plan is to share the learnings and systems with other centers in the U.K. Once established, the systems could potentially be rolled out as a global model for the adoption of advanced therapies.
Cryogenic technology will be crucial to meeting a growing demand for live-cell therapies in the next few years. “I don’t see it as a bottleneck today, but it will become a bottleneck before long,” says Tim Moore, executive vice president of technical operations at Kite Pharma, the company that developed the second gene therapy approved in the United States. “The industry is doing thousands of treatments today. In a few years, we’ll be doing tens of thousands. In the near future, this cryogenic technology is going to be crucial.”





