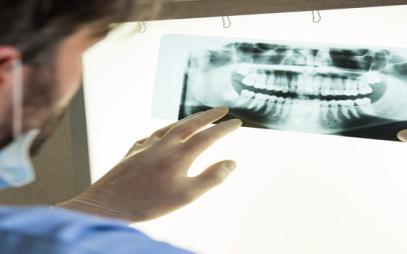
As one of the first to embrace the capabilities of additive manufacturing (AM), the medical industry is one of the pioneers of additive. AM has allowed this industry to increase the quality of life for patients around the world. The orthopedics industry has been leading the way in applying additive technology, from off-the-shelf implants to patient-specific solutions.
Tangible Solutions is a specialized manufacturer of additively manufactured orthopedic implants and a heavy user of Laser Powder Bed Fusion (L-PBF), also known Direct Metal Laser Melting (DMLM). Through Tangible Solutions’ experience, they understand the challenges of new-product development in the additive space and the design and process-control considerations of medical implants.
During this webinar, Stephan Zeidler from GE Additive and Matthew Shomper from Tangible Solutions explore how the company is leveraging DMLM technology to improve patient outcomes and push the limits of design and process control in metal PBF.
Speakers
Matthew Shomper
Director of Engineering
Tangible Solutions, Inc
As director of engineering at Tangible Solutions, Matthew Shomper strives to connect with customers and understand their requirements, helping them to drive toward product launch in the shortest possible timeframe. He has driven the design and development of numerous medical devices, from concept development to market launch, and thus understands customers' pain points, timelines, and budget constraints. With the dual knowledge of both manufacturing and design and development, Matthew is able to swiftly direct projects through V&V (verification and validation) activities and FDA approvals by applying a rigorous understanding of device requirements.
Stephan Zeidler
Senior Global and Key Accounts Director
GE Additive
At GE Additive, Stephan Zeidler serves as a senior global and key accounts director where he oversees medical industry-related strategy and business development activities. He is involved in global account management and product management activities and continues to escort multiple companies - ranging from service providers, OEMs and global medical device manufacturers - on their additive journey. He joined GE Additive in 2016 as global business development manager for the medical sector and has more than seven years’ experience in the medical additive manufacturing industry. Prior to joining GE Additive, Stephan held product and key account management roles in the additive manufacturing industry, working with multiple additive technologies such as PolyJet and FDM.