Jeff McCaulley, CEO of Avalign Technologies, is a passionate advocate of metal additive manufacturing, especially Electron Beam Melting (EBM), in the field for orthopedic implants, where his company is one of the fastest-growing contract manufacturers.
The orthopedics industry was an early adopter of metal additive manufacturing for implants, and growth is expected to continue. SmarTech Analysis estimates that by the mid-2020s, metal 3D-printed orthopedic implants will generate more than $1 billion in revenue opportunities. The industry analyst sees the fastest-growing segments within the orthopedic sector for metal additive are components of knee reconstruction systems, spinal fusion devices and non-load-bearing extremity fracture devices.
Capability, Capacity and Consolidation
“Orthopedic OEMs are increasingly outsourcing and looking to do so with contract manufacturing organizations that have comprehensive capabilities and the capacity and resources to scale up. At Avalign, we’ve built one of the most complete portfolios, covering virtually every component and every step in the value chain of orthopedic manufacturing,” says McCaulley. “We are one of only a few companies that can design, develop, manufacture, and deploy every aspect of implant and instrument systems – including entire programs – in collaboration with our OEM partners. This is very compelling in our industry.”
In line with its commitment to be the leading contract design, development, and manufacturing organization in orthopedics, and to establish a leadership position in its 3D printing capabilities and capacity, Avalign acquired Slice Manufacturing Studios this past January to become its center of excellence for additive and advanced manufacturing.
Slice, now known as Avalign Additive and Advanced Manufacturing is a full-spectrum additive and subtractive manufacturing studio. Located in Akron, Ohio, this 40,000-square-foot, one-of-a-kind manufacturing facility offers services from design, development, prototyping, mechanical testing, and regulatory services to final production and sterile cleaning and packaging.
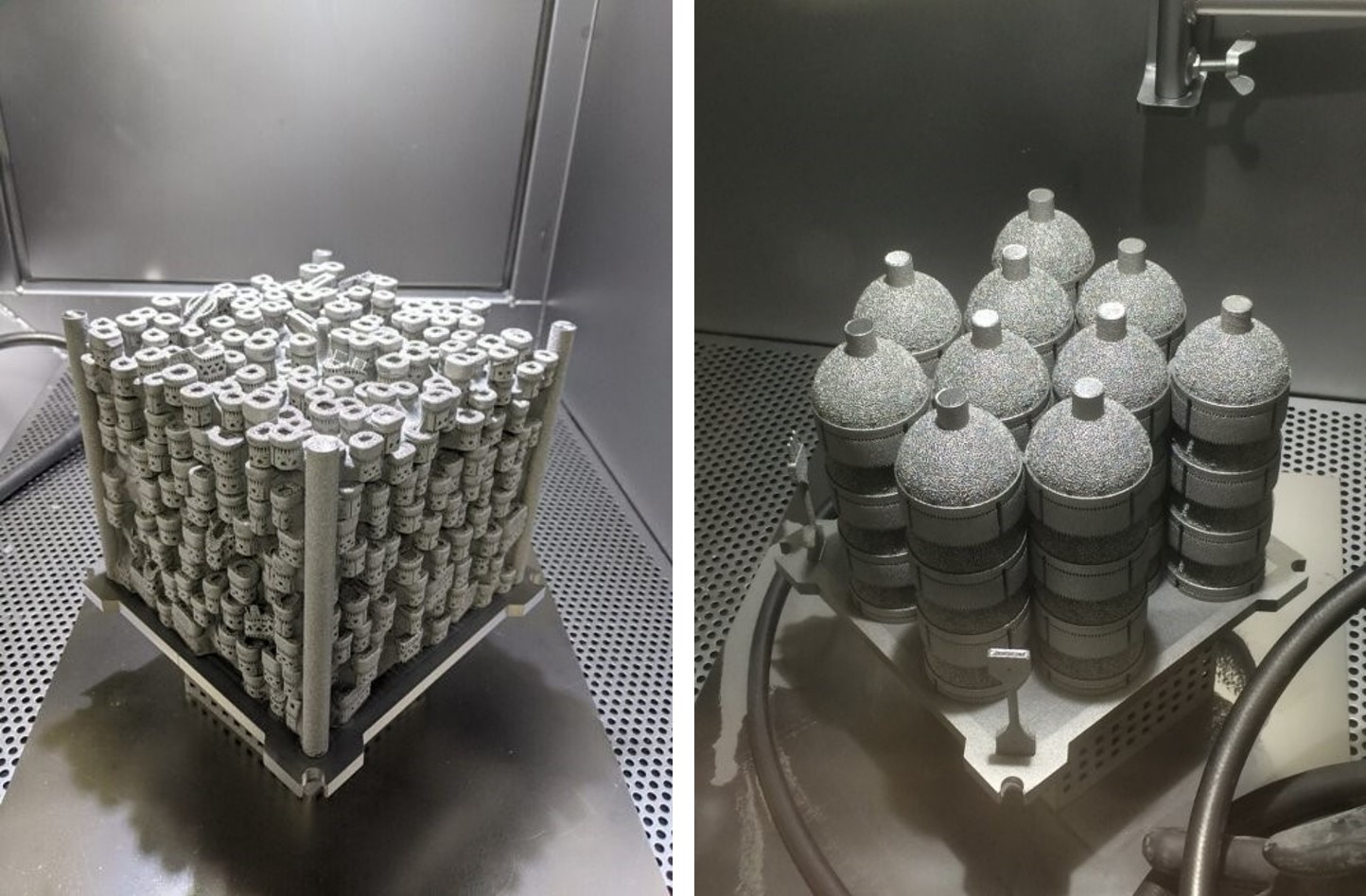
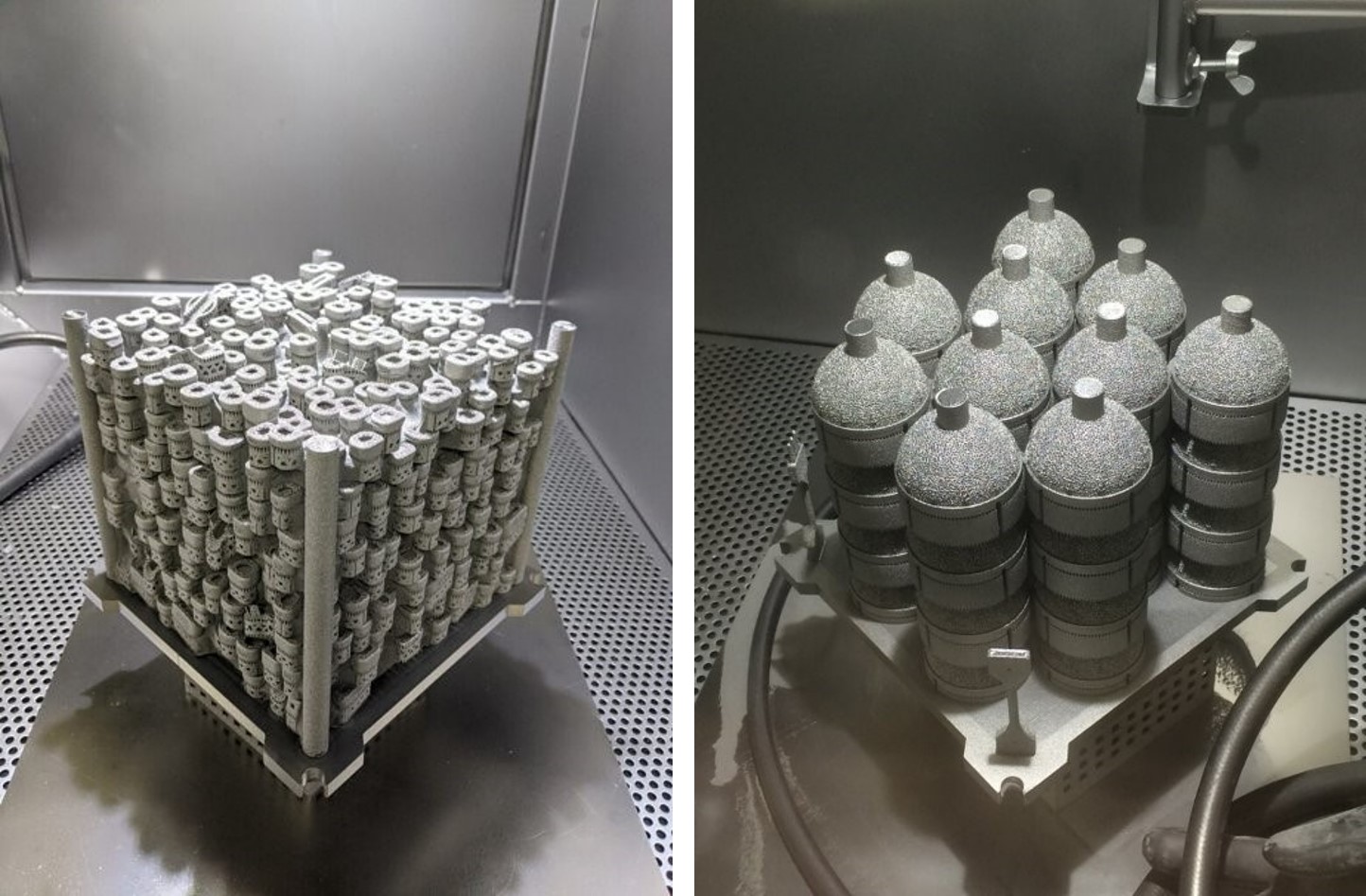
Avalign currently runs eleven GE Additive EBM systems - one of the largest medical manufacturing focused EBM fleet deployments in the world. For orthopedic implants, the build area of the GE Additive Arcam EBM Q10plus is designed to allow for optimal stacking (also known as “nesting”) of the most common implant types, with the build chamber developed for easy powder handling and fast turnaround times. Of the eleven machines running at Avalign, two GE Additive Arcam EBM Q10 machines are used as development machines and to make fixtures and tools.
EBM platform enjoying tremendous success
“The Arcam platform is uniquely qualified for large joint manufacturing, which is not easy with a laser platform. With the Q10plus you can nest your products and stack them on top of each other so that you can best use the time and the powder that's in the machine. It saves us time; it saves us and our customers money. It’s just a very good process for our customer base,” says Allen Younger, business development and product specialist for additive manufacturing at Avalign Technologies.
There's a natural profile that goes along with that customer.
“We're immediately appealing to those who have already accepted that EBM is the best technology for their product and they're looking for a contract manufacturing organization that can scale. We are also finding, however, that customers once predisposed to laser are increasingly seeing the value of EBM,” he continues.
While Avalign is a leader in EBM applications, it also has laser additive capabilities. Moreover, the company says it doesn’t see EBM and laser as being in competition; rather it’s about offering the best tool for each customer application each time. Still Avalign believes EBM offers a differentiated solution for most large joint implants and certain categories of spinal implants. It’s efficient and cost effective with parts that come off the machine nearly complete, saving time and money bringing a product to market.
Great technology needs a great team
EBM, and additive in general, is a next-generation manufacturing technology, but it also needs complimentary technologies and a great team, not only in design and prototyping, but all the way through to the operators running the machines. Avalign has talented engineers with a unique set of skills and Younger can’t highlight enough how they constantly make the company’s machines “sing.”
“From the beginning, we’ve encouraged our teams to embrace complexity, because that is where additive manufacturing really differentiates itself from subtractive manufacturing. There are no additional costs for making a difficult-to-manufacture part. We tell our customers to remove all their constraints, unleash their imagination for solving the most complex clinical challenges, bring them to us and we’ll print it! From a design perspective, engineers are becoming more daring and confident with their designs and run out of their studios telling us ‘I can make this three, four or five different ways!’ It’s amazing to watch!” says McCaulley.
Collaboration across the value chain
In such a rapidly developing field of technology, it’s also essential to have far-reaching collaborations with experts in the field who see cutting edge applications from numerous industries. As such, Avalign works closely with GE Additive. It’s an added-value collaboration of skills sharing and process and knowledge transfer. “We often have the GE Additive team on site, challenging us to do more together, and sharing ideas on how we can push the envelope to better serve our customers,” McCaulley adds.
Experience leads to faster approvals
In the United States, the Food and Drug Administration (FDA) has its own Additive Manufacturing Working Group, and with a better understanding of the technology and its use, many approvals for new orthopedic applications now take only months, rather than years.
That’s exciting news. While orthopedic implants represent some of the most successful medical devices in modern medicine, additive manufacturing has the potential to usher in a new generation of designs.
Accelerating growth in EBM
After a period of impressive growth of metal additive technology in the orthopedic sector, what about tomorrow?
“There are a lot of things that indicate this market is only going to grow. It’s a question of how much faster orthopedic OEMs, contract manufactures like Avalign, and the additive machine manufacturers like GE Additive can continue to innovate? The promise of personalized implants has long been seen as the end game. While 3D-printed, patient-specific implants are not yet approved by the FDA, the potential is promising and could greatly propel the growth in AM for orthopedics,” says McCaulley.
Additive experts are beginning to enter operating theaters to guide surgeons – a development indicative of the collaboration throughout the additive value chain from healthcare and medical professionals, the device and implant designers, the contract manufacturers through to the machine OEMs.
“I'm quite excited about what's happening in orthopedics today. Some of the applications are just phenomenal, but I’m even more excited about what we can expect for patients in the future and especially about our role in the value chain,” adds McCaulley.